Allele Biotech has dedicated three years to the development, verification, and validation of cGMP iPSC SINGLE CELL cloning. Following successful cGMP operations for internal projects and CDMO projects for our pharmaceutical partners, we have compiled these brief technical notes to help others working on similar projects at various stages.
1. iPSC Source
The starting iPSC property has been identified as the most critical parameter. For each iPSC line, it is highly recommended to conduct a pre-process development step, even if brief, to finalize the process parameters tailored to that specific line before advancing to the GMP stage.
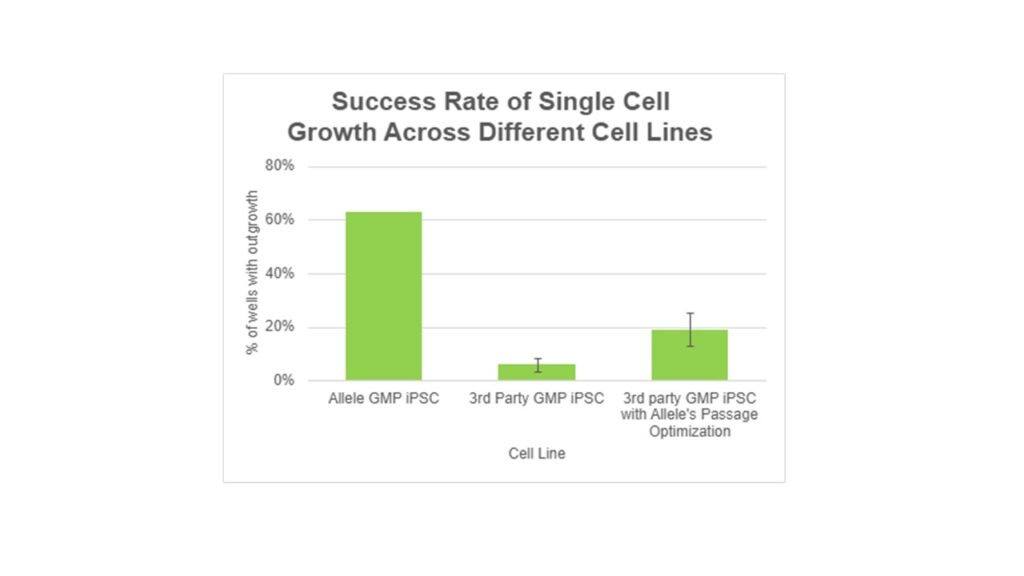
To illustrate why the intrinsic properties of the starting iPSC line are so crucial, we present a real case study below.
For Allele’s iPSC lines, established through mRNA reprogramming, the typical success rate after single-cell plating is approximately 50 usable wells from each 96-well plate. With 16 wells allocated for various controls, the effective success rate is 50 out of 80. In contrast, some iPSC lines from partner or third-party sources, which were less completely reprogrammed or improperly maintained, yielded only 1-5 wells with successfully established single-cell clones using the same process. However, with our pre-process optimization, such as passage numbers before single cell selection, this success rate can be improved to 10-20 wells.
2. Plating Method
During the method comparison phase of GMP development, we considered and tested several options, including conventional serial dilutions, FACS with well indexing and Single Cell Dispensing (in our case, using a Cytena C.SIGHT instrument), which we ultimately selected . Despite lower throughput than FACS, the high viability of the cells and the image-based proof of monoclonality led us to a rapid adoption of Single Cell Dispensing technologies.
During the early method development for using this platform, Allele’s iPSC lines initially achieved approximately 30 wells per 96-well plate consistently using the Cytena platform. In contrast, Extreme Dilution—a proprietary protocol we refer to internally as ‘Manual Plating’ —yielded around 10 wells per 96-well plate. Conventional serial dilution consistently demonstrated the lowest efficiency across all metrics, and is the reason why we do not recommend its use. Other instrument-based platforms we tested either had a lower success rate (though still better than serial dilution) or required a longer recovery period.
3. GMP Process
Allele’s commitment to GMP development goes beyond merely offering iPSC lines labeled as ‘GMP compliant.’ Our deeper motivation, rooted in cGMP development and mRNA reprogramming since 2014, lies in the understanding that cGMP encompasses more than just cleanrooms and documentation. At its core, GMP is centered around Quality by Design (QbD).
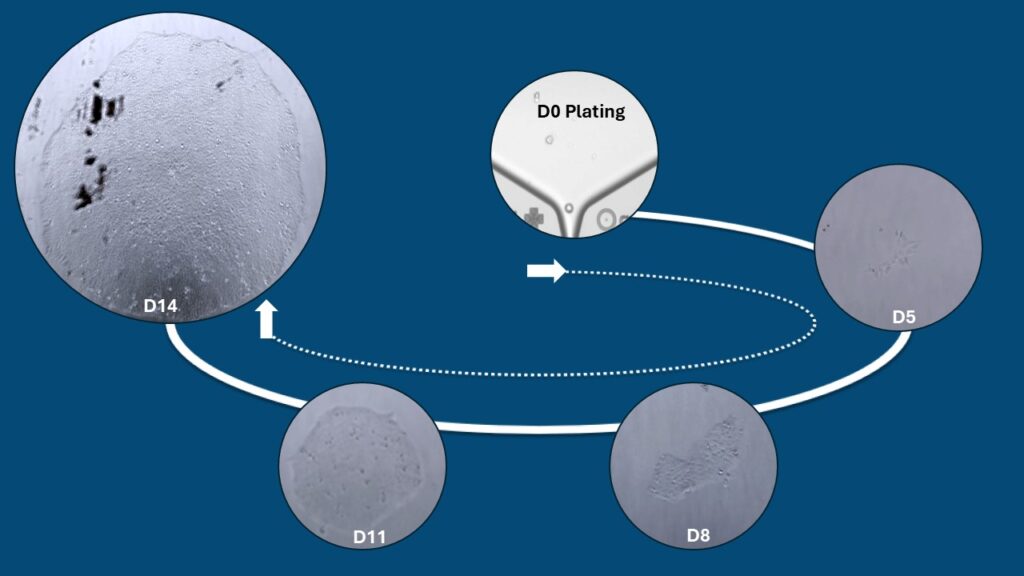
While plating is the most critical step in single-cell selection, the complete process also involves thawing the starting cells and maintaining cultures until they meet pre-established criteria for suitability. During the plating procedure, various settings and criteria are applied to ensure that the selected wells contain only a single cell. Post-plating, various parameters are identified, and culture conditions are optimized and standardized to maximize colony formation in terms of a high success rate and quick recovery period.
As noted above, our GMP operations currently achieve an approximate success rate of 50 out of 80 wells, an improvement from around 30 out of 80 prior to completing the GMP process development phase.
4. Proof of “Single Cell”
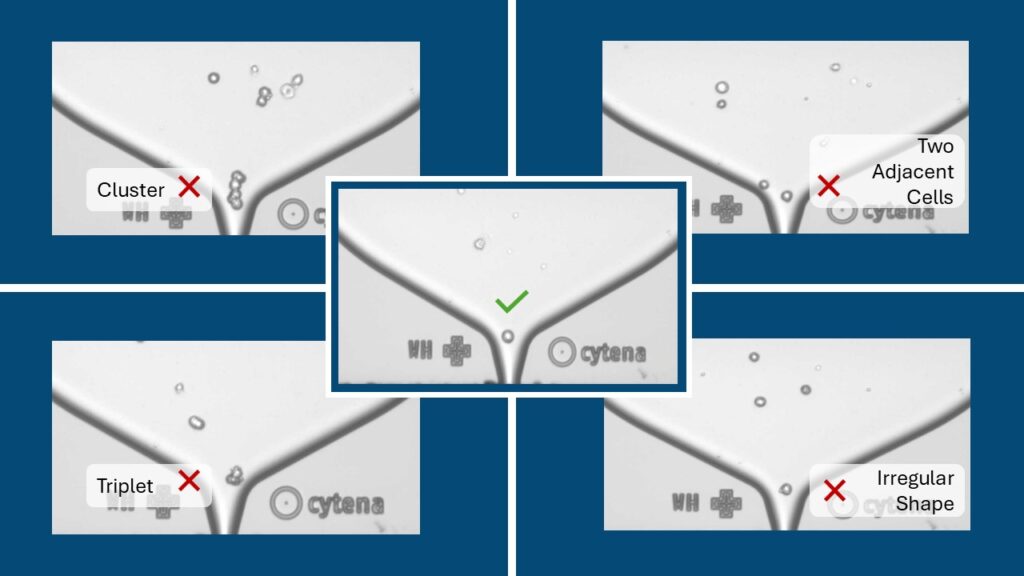
Establishing cell lines from a single cell may not be essential for general iPSC line usage; however, after genome editing, it becomes necessary to employ a single-cell selection process to ensure that the iPSC population has a uniform edited genome. While whole genome sequencing and other testing methods can identify differences at the single-cell level, they cannot be used to select a specific single-cell clone based on these differences. In our single-cell dispensing process, the detection of cells and other objects within the nozzles of the drop-on-demand dispenser relies on microscopy and automated image processing to identify droplets containing the single cell. These images can be stored for later analysis and serve as proof that a single cell was indeed dispensed into the target well. The video below demonstrates our process using the Cytena C.SIGHT platform.
5. GMP Capacity
The challenge of simultaneously maintaining multiple single-cell clones under cGMP conditions is commonly overlooked and underestimated. This is because after plating, each clone is considered an independent cell line and must be managed separately with established measures preventing crossline mixing.
Closed systems and automation at this stage can significantly alleviate the burden, and at Allele, we have dedicated resources to their development. We have optimized a cryopreservation protocol that ensures a high recovery rate, even when cells are preserved at extremely low counts. This advancement enables the large-scale cryopreservation of single-cell-derived iPSC lines, accommodating hundreds of lines for screening, all under cGMP conditions. Each clone from this process will be stored in two vials: one vial can be transferred to a research lab for identification, while the other will be reserved under cGMP compliance.
